tel
400-1818-529
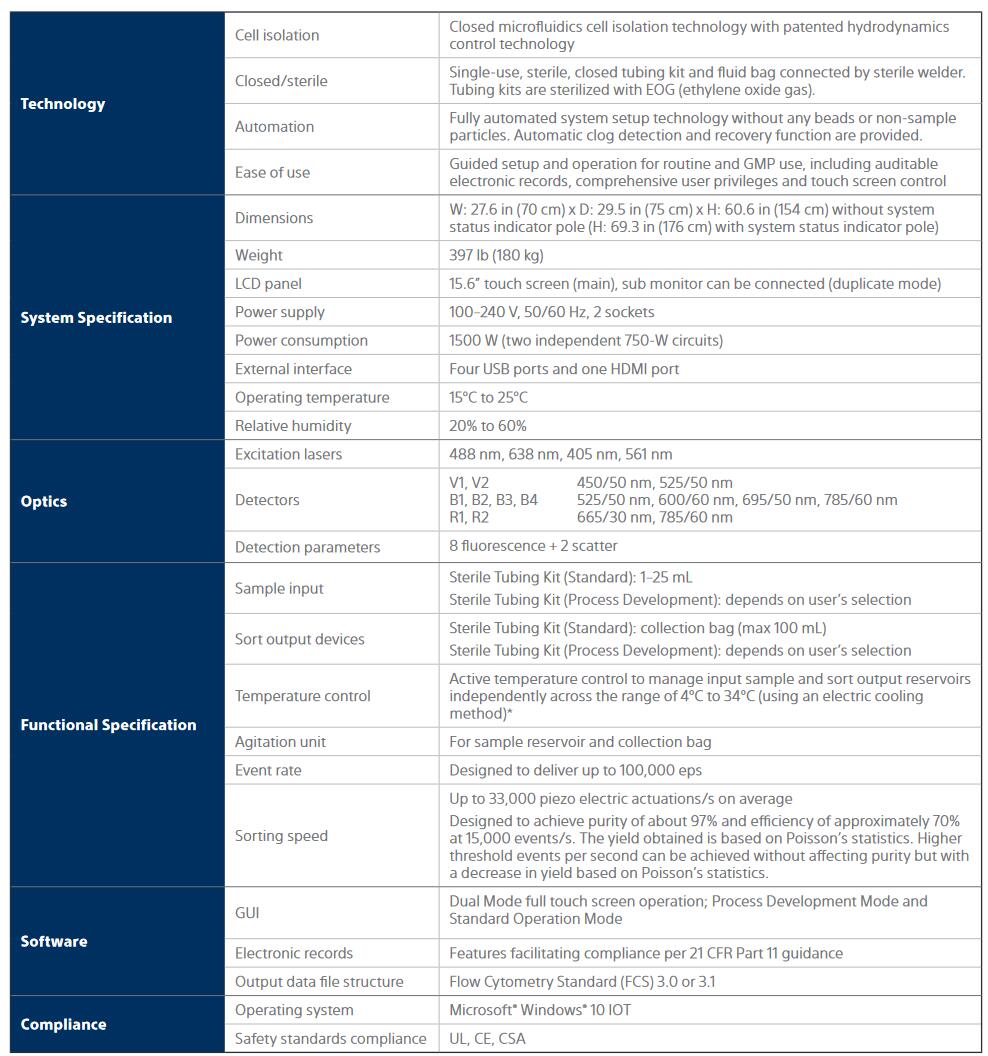
Progress in the area of cell and gene therapies has accelerated significantly in recent times. Flow cytometry-based cell sorting has been used to sort cells by desired phenotype based on the presence of one or more biomarkers. Sorted cells have been expanded ex vivo and reinfused into patients as cell-based therapies. The ability to use multiple biomarkers is important. It enables identification and isolation of specific immune cells possessing the desired phenotype. Multi-marker-based cell isolation not only delivers a higher purity of isolated cells, but also enables selection to eliminate non-target cells, or therapeutically ineffective cells, for example, over-differentiated T cells.
However, the use of traditional flow cytometers has been limited to research laboratories and preclinical and early phase studies. The complex design of conventional flow cytometers and their usability issues makes them poorly suited to a cell production environment that is GMP compliant. Certain conventional flow cytometers have also been known to shear or cause electrically-induced stress or damage to sorted cells.
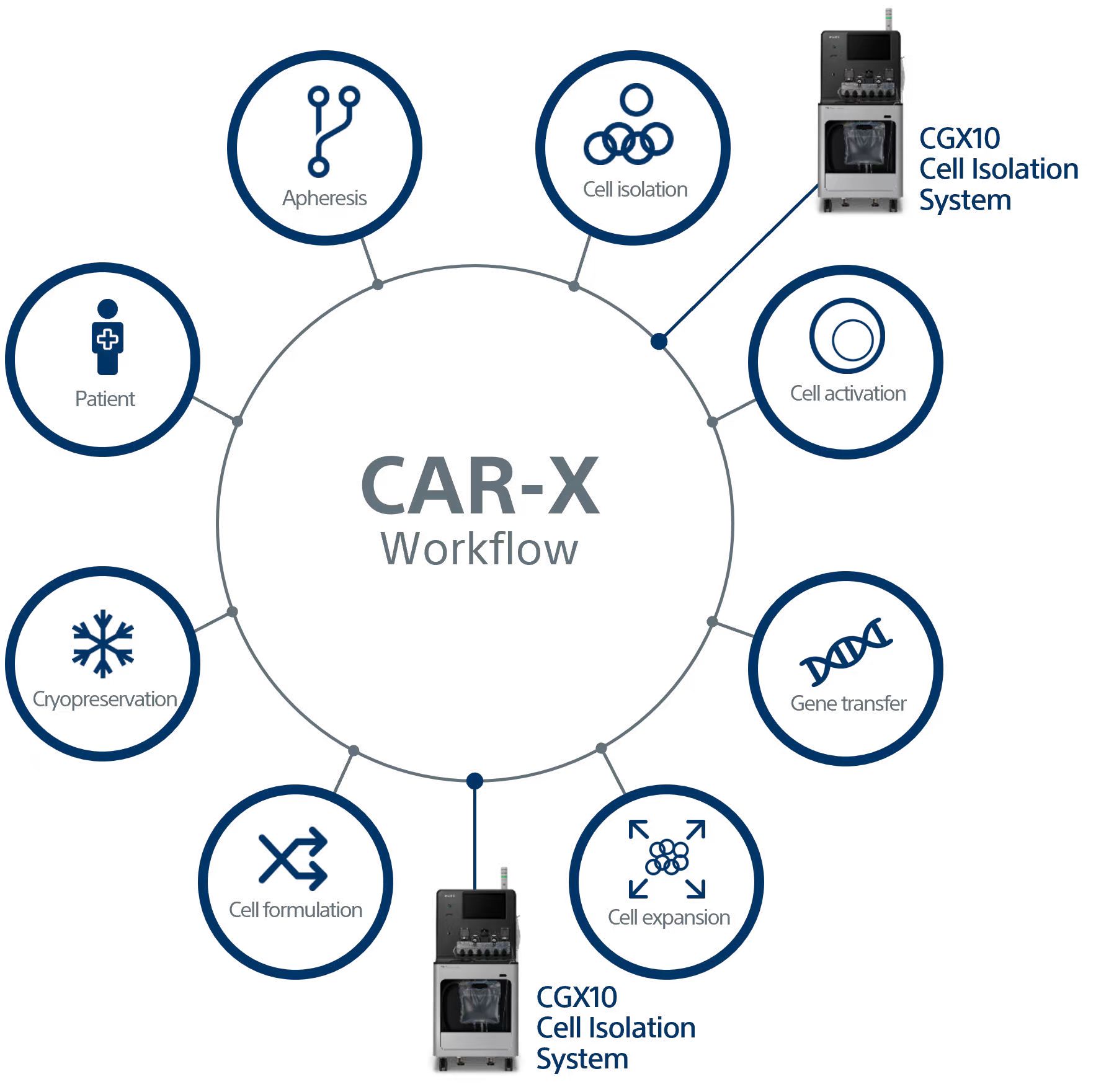
The CGX10 Cell Isolation System has been developed with the goal of enabling multi-marker selection to isolate target cells while ensuring the highest cell viability. The system delivers a user experience that is suited to the GMP compliant, cell production environment, and transcends the complexity and subjectivity of conventional sorter platforms.

The CGX10 Cell Isolation System and related products are intended for use by trained laboratory technicians in research, process development, or manufacturing environments all related to ATMP/regenerative medicine, including cell and gene therapy. The CGX10 instrument and related products are for ex vivo cell separation processing only, and are not intended for therapeutic, diagnostic, or human in vivo applications. Any clinical application of the cells is exclusively within the responsibility of the user of the CGX10 instrument and related products. For the manufacturing and use of cells in humans, regulations must be followed. The CGX10 Cell Isolation System and related products are not sold as medical devices.
1. Closed structure that maintains sterilization
The product features Sony’s proprietary closed sorting structure which maintains a sterile state and completes cell isolation inside a chip. Within the chip, after the cell has been analyzed, the selected cell will be isolated by controlling the flow of the liquid.
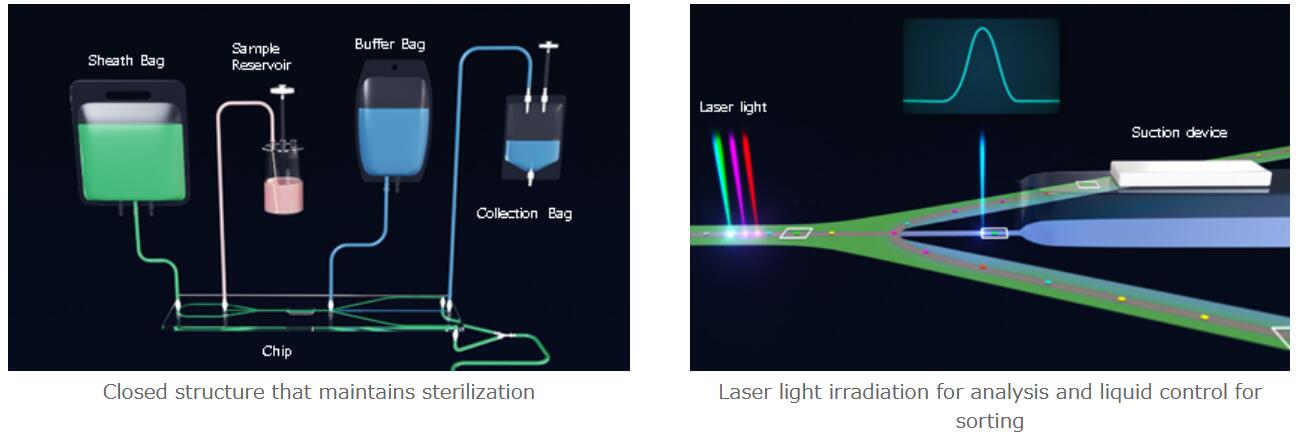
Closed structure that maintains sterilization
2. High-speed, high-purity, high-viability cell sorting
Based on use of up to ten parameters (cell markers) obtained from excitation using four lasers (405nm / 488nm / 561nm / 638nm), the instrument can sort a massive number of cells at high speed and with high purity. Furthermore, the user can change the priority settings for sorting. With the purity priority setting, purity will reach about 97%, and with the speed priority setting, the speed can reach roughly 100,000 cells analyzed per second. As a result, it achieves a level of productivity suitable for the mass production of cell and gene therapy manufacturing.
In addition, cell damage is minimized by proper control of the liquid. This ensures a high viability of cells, which increases efficiency in the next stages, such as gene transduction or culturing of the isolated cells.
3. High operability
The CGX10 instrument is equipped with a touch LCD touch display panel allowing seamless and intuitive operation of the hardware alongside the software. In addition, there are two types of software modes: the “process development mode” and the “standard operation mode.” In the “process development mode,” detailed settings and customizations are possible, allowing the user to create instruction sets. The “standard operation mode” will automatically sort cells based on the instructions set in the “process development mode.” Multiple instruction sets can be developed on a given instrument and transferred to multiple instruments for production. “Standard operation mode” includes functionality suitable for GMP manufacturing and has a function to record operation history.
4. Employing a single-use disposable tubing kit
The tubing kit includes a plastic tube specifically made for controlling the liquid that comes in contact with the sample. This single-use disposable tubing is intended to be replaced after each use. A new tubing kit can be used for each patient, avoiding contamination between samples.
5. Documentation support for GMP manufacturing
This instrument is designated for use in cell-based immunotherapy settings including gene therapy, as well as cellular manufacturing for GMP regenerative medicine. Sony will provide user support by distributing the compliance support documentation necessary for GMP manufacturing, such as the CoA (analysis certificate), animal-free production certificate, and product information files.